Answer of January 2015
For completion of the online quiz, please visit the HKAM iCMECPD website: http://www.icmecpd.hk/
Clinical History
A 38 years old lady with unremarkable past health presented with progressive exertional dyspnea for 1 month. Physical examination revealed elevated jugular venous pressure, bilateral lower limb oedema, bilateral basal crepitations and ascites. Chest X ray showed presence of cardiomegaly, bilateral pleural effusion and congestive lung fields.
Cardiac MR was requested for further evaluation of her condition.
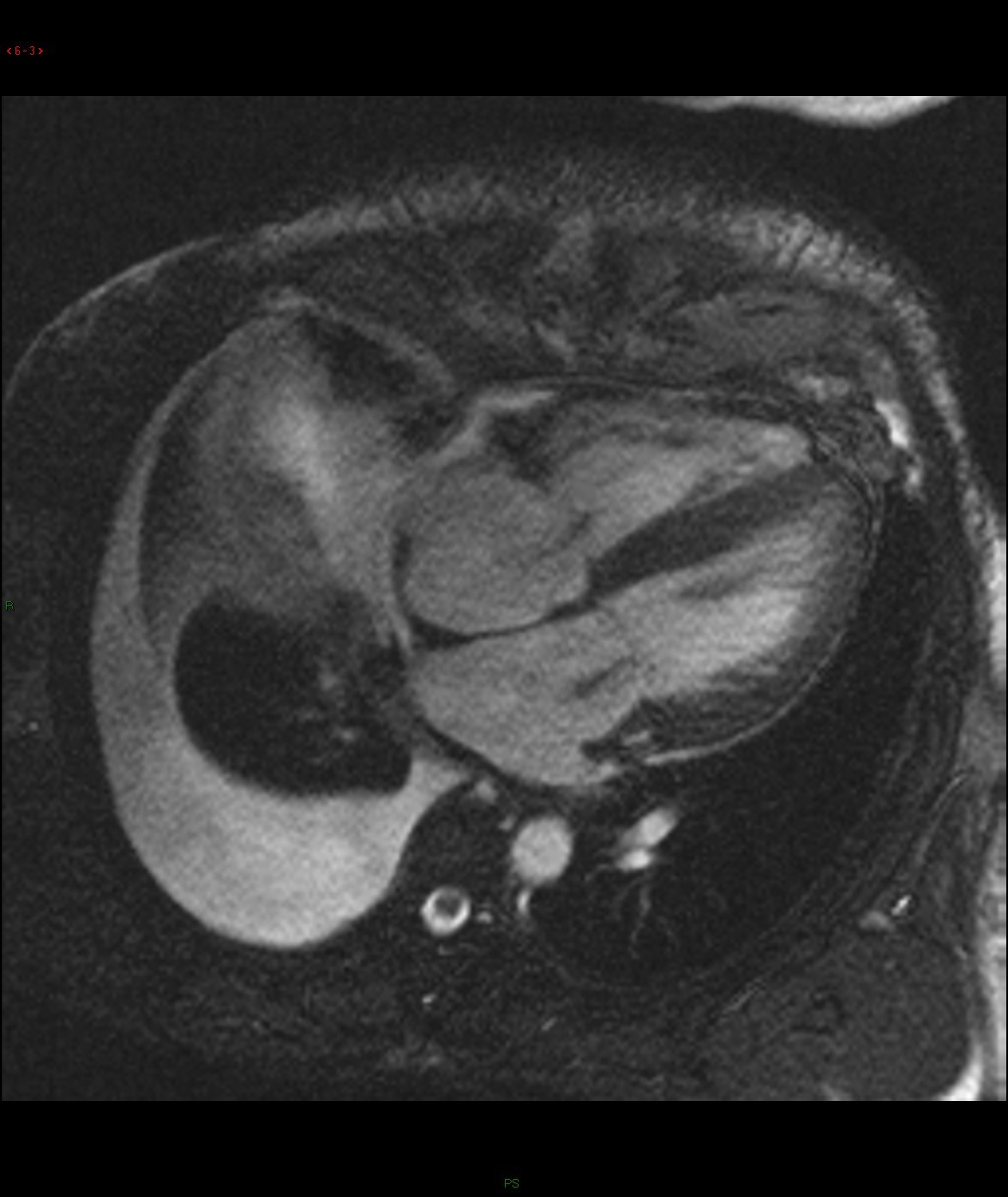
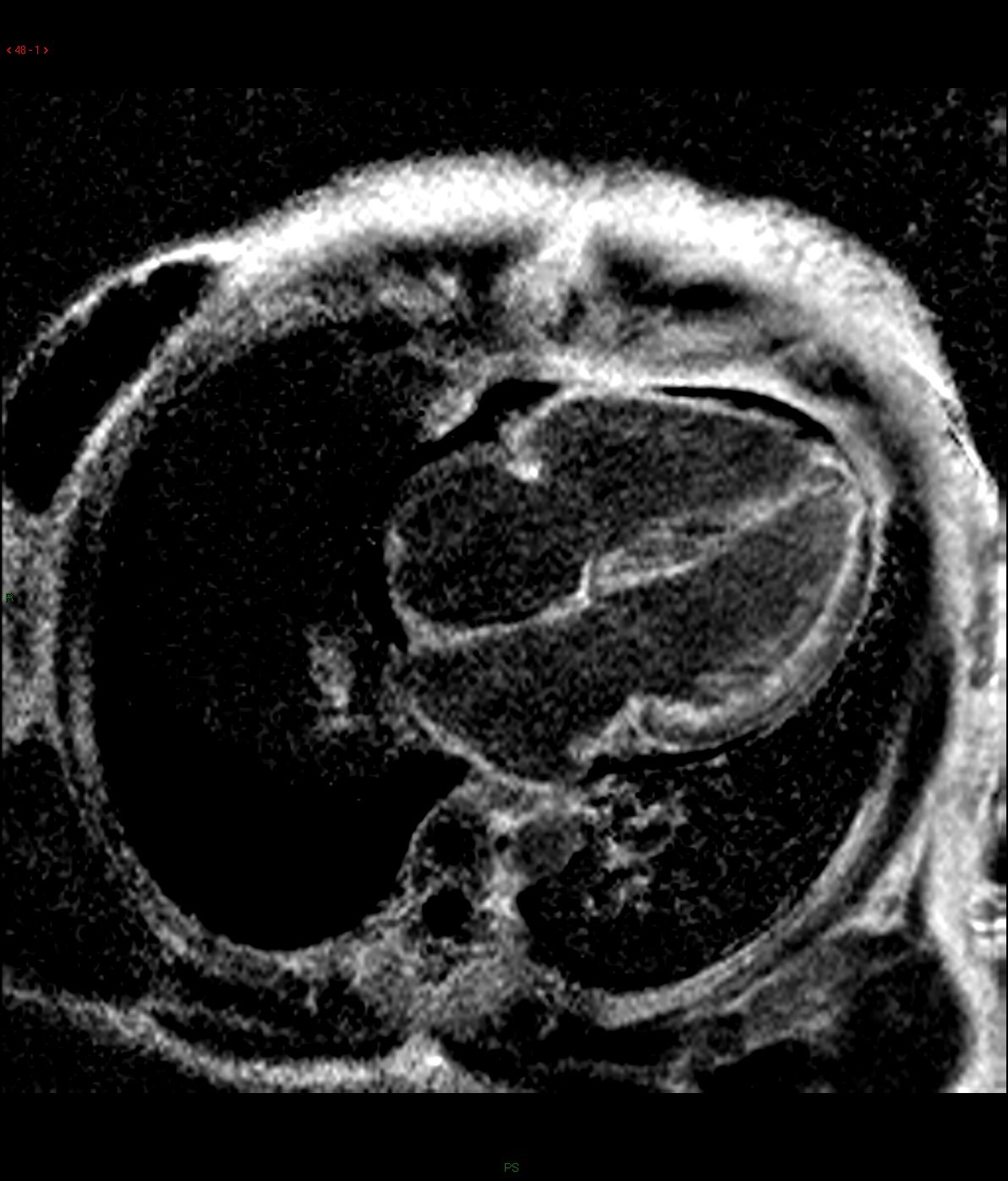
Selected images from CINE: 4-chamber and basal short-axis
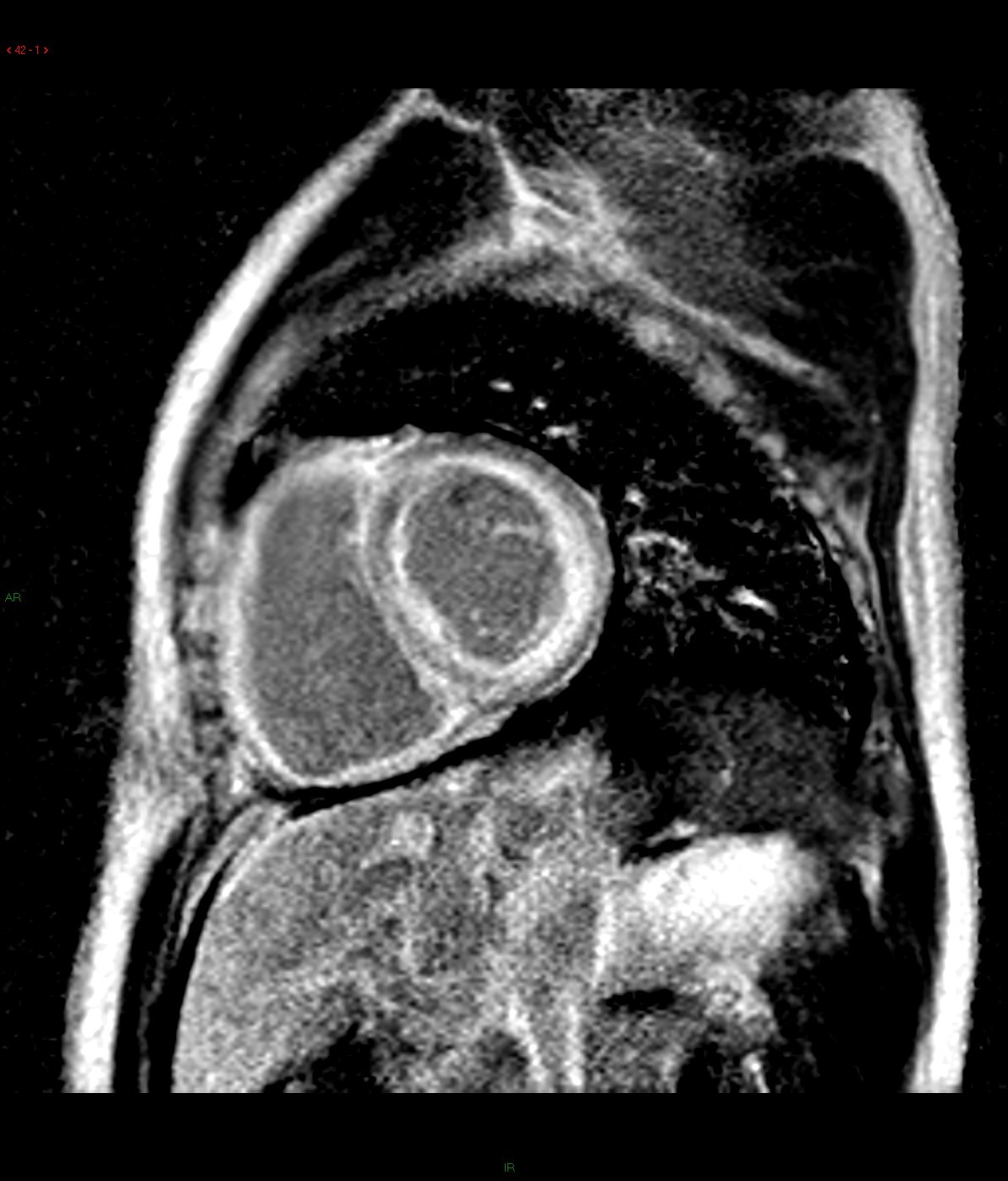
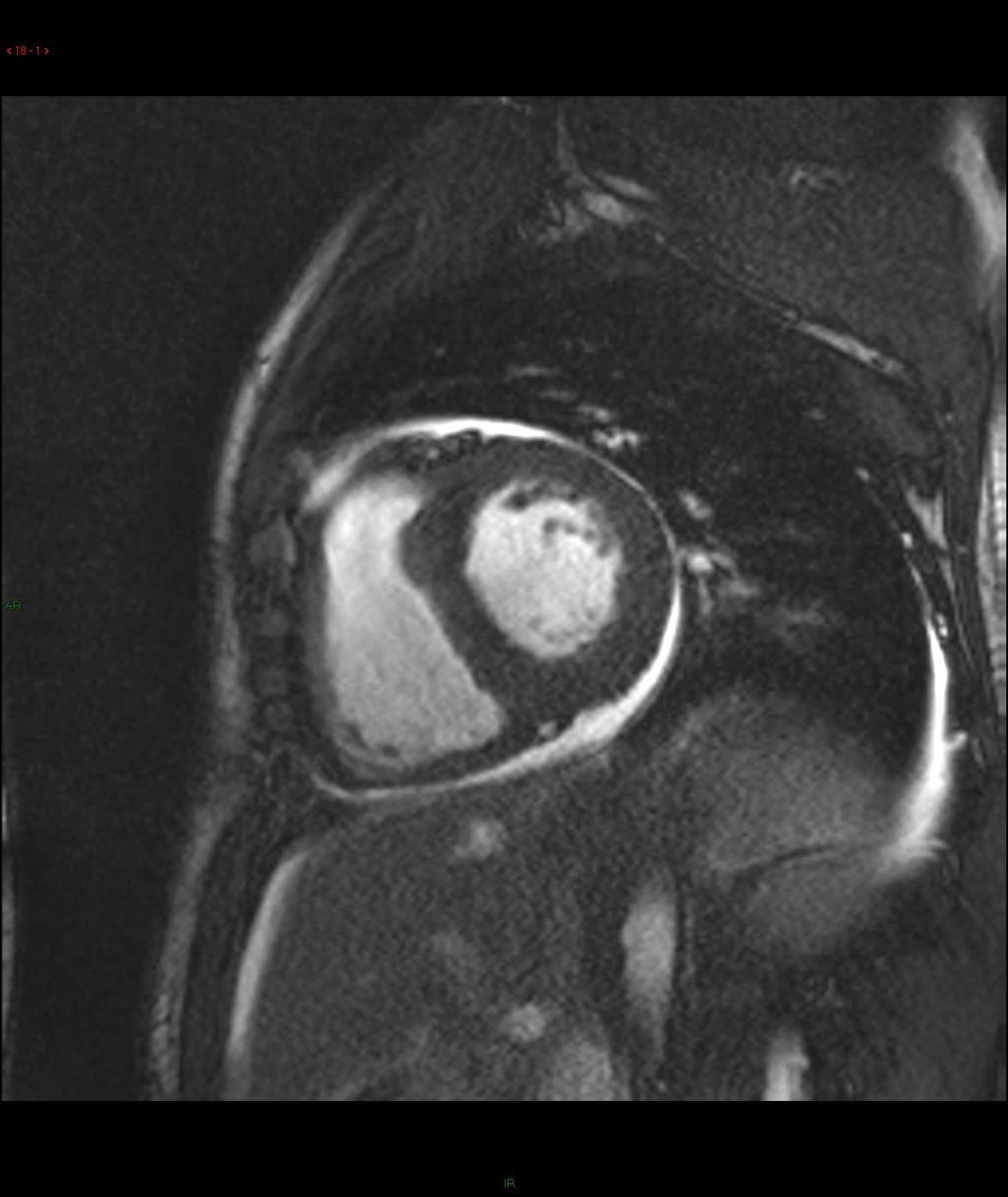
Late gadolinium enhancement: 4-chamber and basal short-axis
Diagnosis:
Cardiac amyloidosis.
Discussion:
Cine images of cardiac MR demonstrated global left ventricular hypokinesia (ejection fraction 30.7%) with restricted diastolic filling. Mild thickening of the both ventricular wall is evident. There are mitral and tricuspid regurgitation with biatrial enlargement. Diffuse subendocardial late gadolinium enhancement is seen. There is also evidence of right pleural effusion. Overall imaging features are suggestive of cardiac amyloidosis.
Amyloidosis is caused by an extracellular deposition of insoluble amyloid proteins in the myocardial interstitium. It may occur as a multisystem disease or a localized disease with single system involvement. With cardiac involvement, it diffusely thickened the myocardium and manifests as a form of restrictive cardiomyopathy.
For cardiac amyloidosis, MR will reveal a diffuse myocardial thickening, with involvement of the interatrial septum and right atrial free wall. Impaired systolic and diastolic functions are usually evidence. A diffuse subendocardial late gadolinium enhancement of the left ventricle is typically seen, which is related to the distribution of contrast material in areas of amyloid protein deposition in the subendocardium. Absence of residual contrast agent in the blood pool has also been described, which is related to retention of gadolinium in the extracellular space throughout the body secondary to systemic amyloidosis. Deposition of amyloid protein causes T1 prolongation and hence difficulties in nulling the myocardium on late gadolinium enhancement images.
Over the years, cardiac MR has gained increasing popularity in the diagnosis, risk stratification and management of patients with various cardiac conditions. It provides detailed assessment of cardiac morphology, function, perfusion and tissue characterization in a non-invasive and radiation-free manner. Late gadolinium enhancement is an important tool in both evaluation ischemic and non-ischemic cardiomyopathies.
In ischemic cardiomyopathies, late gadolinium is helpful in evaluating the presence and extent of myocardial fibrosis, which predicts the likelihood of recovering contractile function following coronary revascularization. Late gadolinium enhancement in ischemic cardiomyopathies is typically subendocardial or transmural and follows a vascular territory.
In non-ischemic cardiomyopathies, late gadolinium enhancement may help in narrowing the differential diagnosis and also provide prognostic information. Late gadolinium enhancement in non-ischemic cardiomyopathies is typically in a non-vascular territory distribution and can affect the subendocardium, mid-wall and/or subepicardium. Cardiac amyloidosis and Loeffler endocarditis (hypereosinophilic syndrome) are two conditions that give a diffuse subendocardial late gadolinium enhancement. A mid-wall late gadolinium enhancement is most commonly seen with dilated cardiomyopathy and hypertrophic cardiomyopathy. When late gadolinium enhancement follows a subepicardial distribution, myocarditis is the top differential diagnosis. Cardiac involvement of sarcoidosis is frequently patchy or nodular in distribution.