Answer of January 2023
For completion of the online quiz, please visit the eHKAM LMS website or HKAM iCMECPD website.
Clinical History:
A 71-year-old man, ex-smoker, complained of four limb weakness for a year, with progressive worsening, upper limbs affected more than lower limbs, urinary incontinence, mild visual blurring and frequent falls. Physical examination revealed hyperreflexia in the upper limbs and spasticity in the lower limbs and with generalized hyperreflexia, power is preserved. Blood tests showed anti-myelin oligodendrocyte glycoprotein (MOG) weakly positive and neuromyelitis optica (NMO) negative. Overall treated as anti-MOG related neuromyelitis optica spectrum disorder (NMOSD). Fair response to steroid and rituximab with progressive symptom worsening with reduced lower limb power. Rechecking of the anti-MOG came back negative. Repeat MRI thus done. DSA followed.
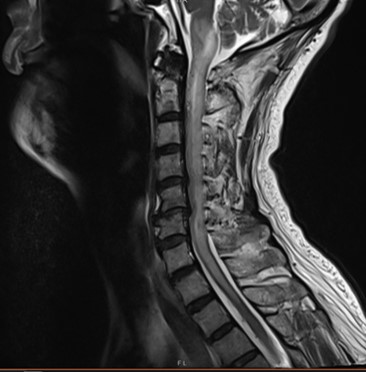
MRI C spine T2 Sag on first presentation
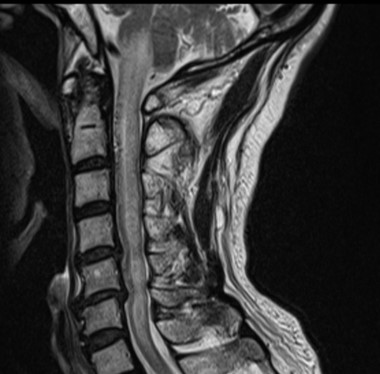
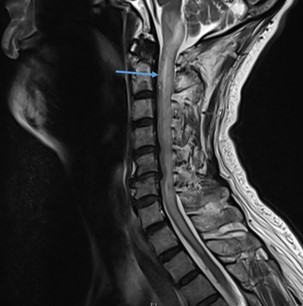
Post treatment MRI C spine T2 Sag
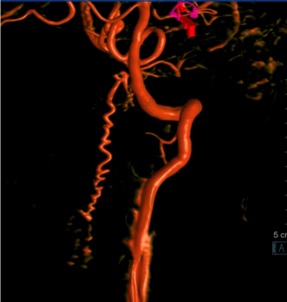
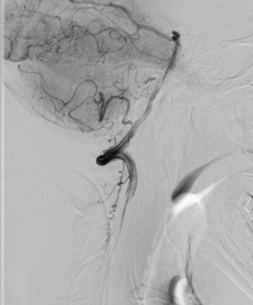
Left vertebral artery DSA angiogram
DIAGNOSIS
Spinal dural arteriovenous fistula
DISCUSSION
Initial MRI C spine showed extensive T2 hyperintense signal in the cervical cord with cord expansion.
Post treatment MRI C spine with annotation
Latest post treatment MRI C spine T2 Sag was done showing increase in T2 hyperintense signal in the cervical cord with progressive edema to the medulla over a year on MRI. It also revealed increased serpiginous flow void over anterior surface of cervical cord on top of cord edema.
DSA later
performed showed abnormal tortuous vessels over left anterior and lateral
aspect of the spinal canal at craniocervical junction, concerned for underlying
dural AVF.
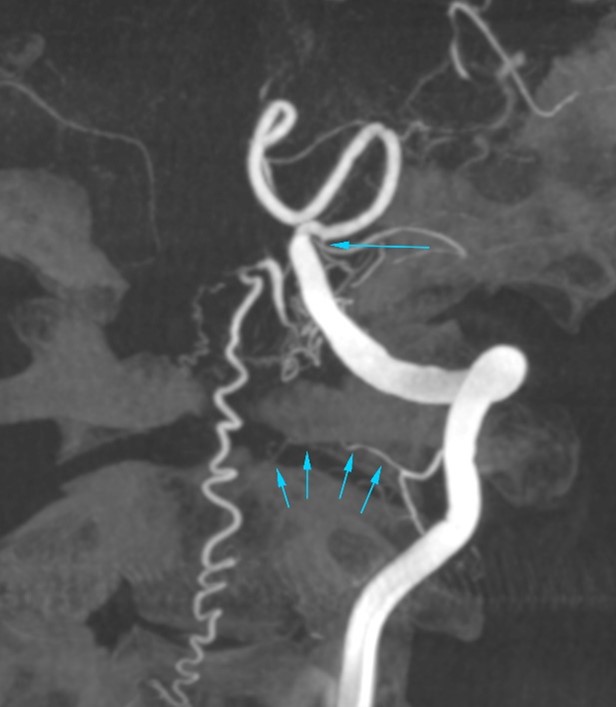
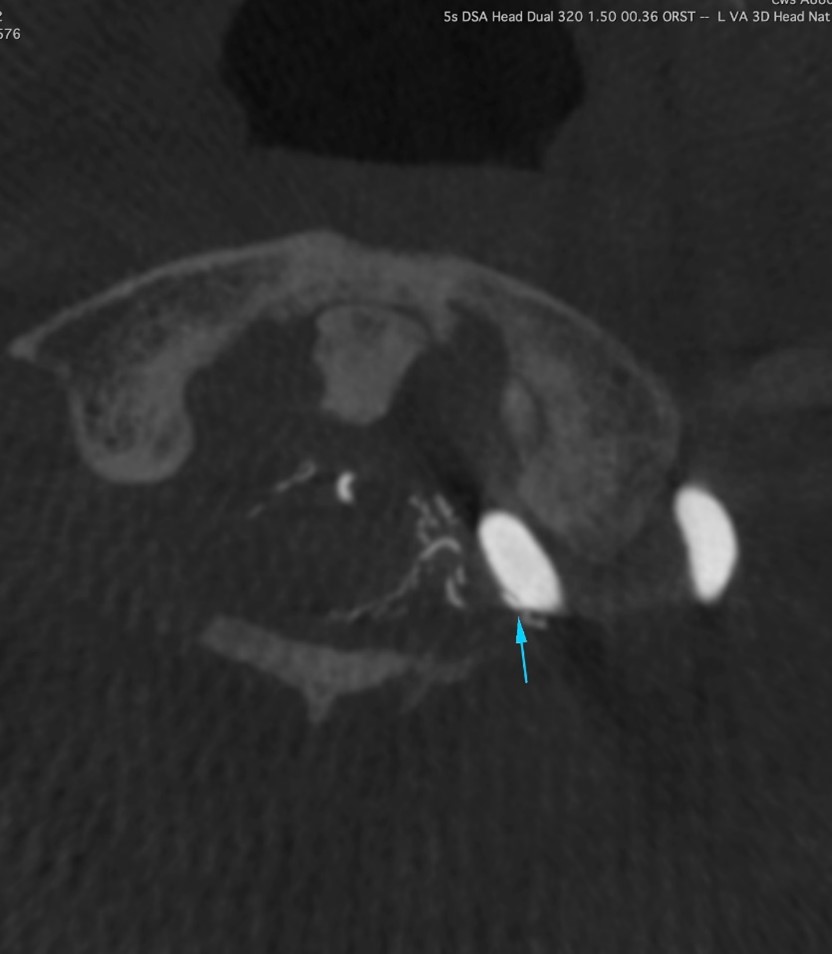
Additional cone beam CT and DSA angiogram of left vertebral artery
Major arterial supplies are suspected to be as follows:
- tiny branch arising from left vertebral artery (VA) at C1/2 level, just proximal to the dural indentation (multiple tiny arrows);
- another tiny branch from the undersurface of the proximal left posterior inferior cerebellar artery (PICA) (long arrow);
-Suspicious minor contribution from a segmental branch of upper left VA at C2 level.
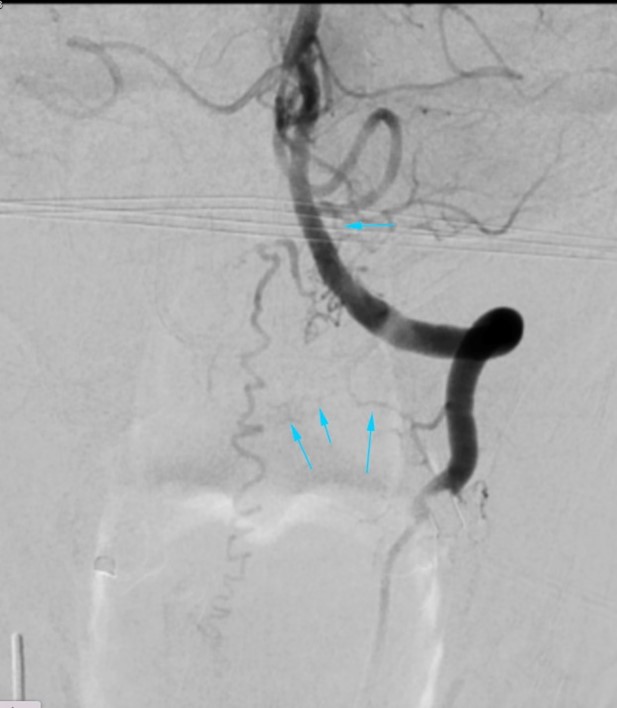
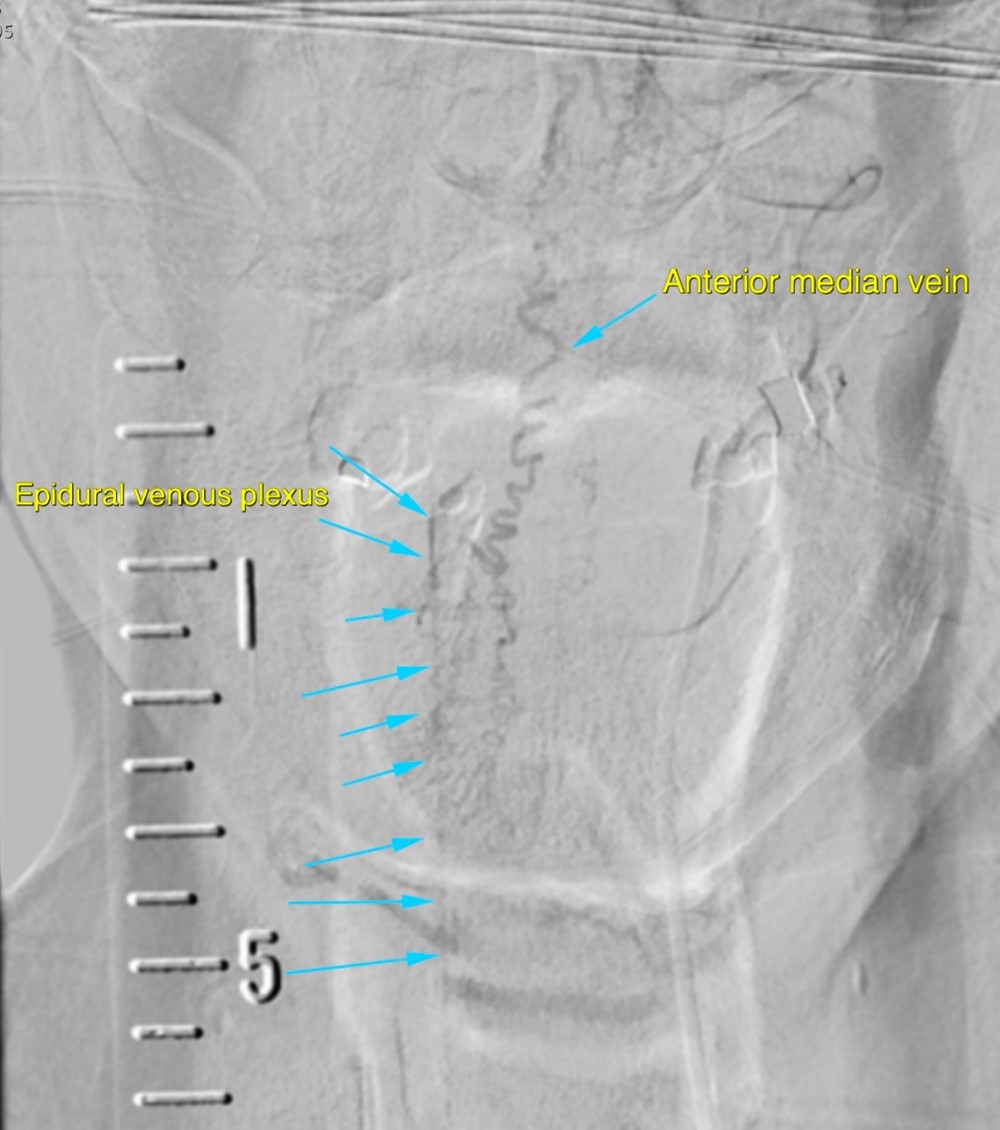
Left vertebral artery DSA angiogram with emphasis on venous drainage
Subsequent venous drainage is noted into the anterior median vein then caudally into the epidural venous plexus.
Symptoms of spinal dural AVFs are due to venous hypertension and cord edema and congestion. Clinical presentations include lower limb weakness, pain, sensory loss and sphincter dysfunction. There is often a significant delay from presentation to diagnosis as in this case.
Imaging includes MRI and the gold standard would be DSA as in this case.
A number of classification systems for spinal arteriovenous malformation exist and two main types would be AVM and AVF. The simplest classification system is based on the radiological appearance and they are classified as follows:
Type I- fistulas between dural branch of spinal ramus of the radicular artery and intradural medullary vein;
Type II- intramedullary glomus malformations;
Type III- extensive juvenile malformations, expanding into the extradural space and involving multiple feeding arteries;
Type IV- intradural perimedullary AVF.
The one described in our case would thus be categorized as type I according to this classification.
Other types of classifications would categorize AVFs as either extradural or intradural, and dorsal or ventral if intradural; and AVMs also subdivided according to their location including extradural and intradural and further into intramedullary, intramedullary-extramedullary and conus, if they are intradural.
Classification system would aid in the understanding of the anatomical location and thus offer guidance for treatment planning.
Treatment options mainly include surgery and endovascular treatment, either one or both with determining factors including location and size.
Endovascular treatment is by occlusion with agents e.g. glue or Onyx. Surgical treatment options include surgical occlusion with laminectomy and intradural exploration with disconnection or coagulation of the draining vein.
The site of maximal MRI abnormality is not a reliable indicator for the location of the fistula and can be many levels away. Therefore, a complete spinal angiogram should include all the possible contributors.
CT myelography could be an alternative if MRI is contraindicated as it may show dilated veins as tortuous filling defects. CT angiography is also an alternative.