Answer of August 2020
For completion of the online quiz, please visit the HKAM iCMECPD website: http://www.icmecpd.hk/
Clinical History:
This 61-year-old man was a heavy drinker and had severe fatty liver. He was found to have hypoalbuminemia (18g/L). The 24-hour urine protein was within normal range. A nuclear medicine scan was performed.
Imaging Findings:
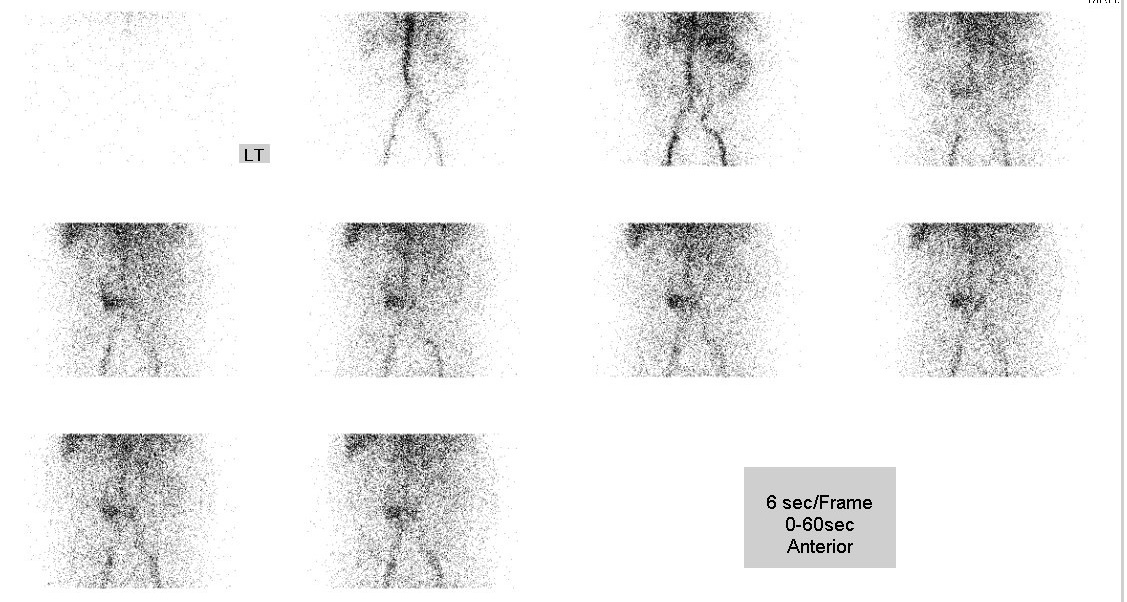
• Perfusion phase: focal increased perfusion is seen in central abdomen (predominantly on the right side of aortic bifurcation)
• Dynamic scan (1-hour duration): mild increased tracer activity in central abdomen;
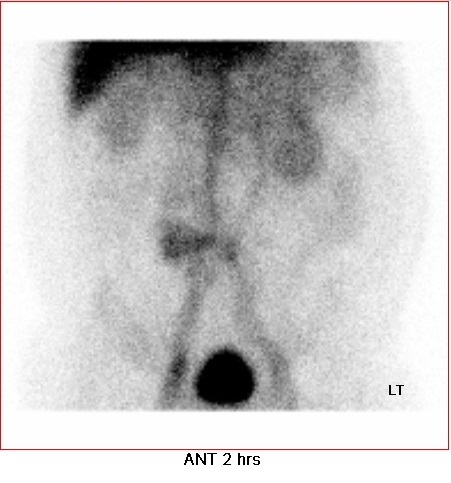
• Delayed static image at 2 hours: focal mild increased tracer activity at central
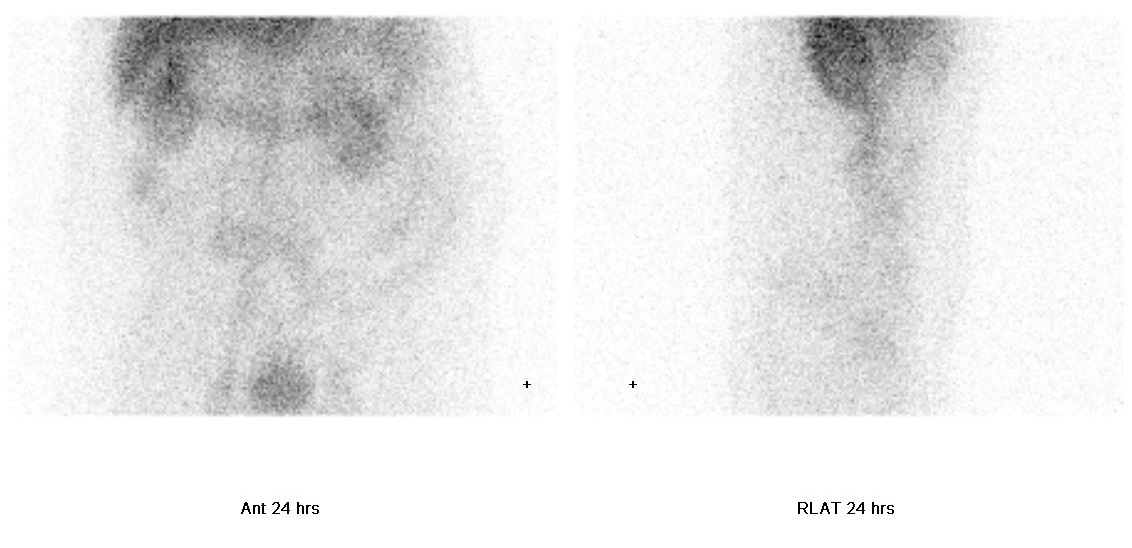
• Delayed static images at 24 hours: focal mild increased tracer activity in right abdomen; mild increased tracer activity is again seen in central abdomen.
Comment:
- Protein-losing enteropathy at or proximal to ascending colon is suspected.
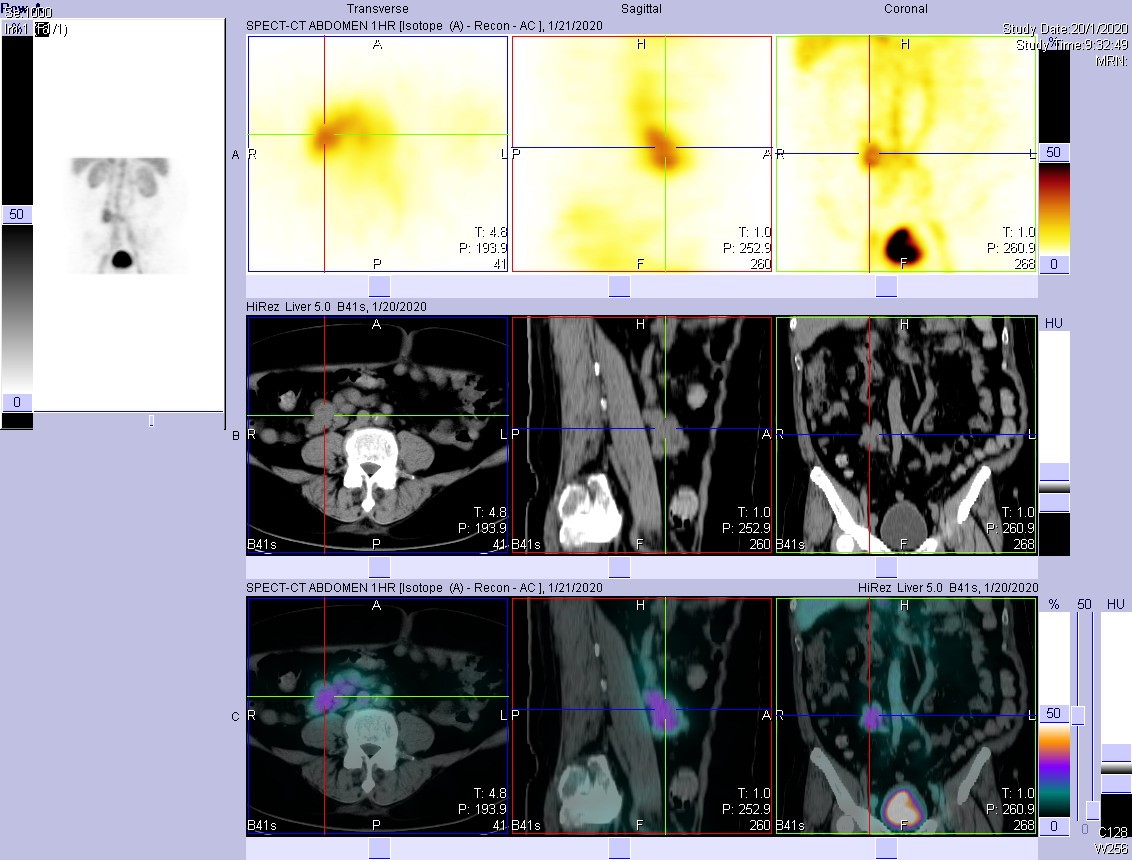
- SPECT/CT revealed that the increased tracer activity in central abdomen is a vascular shunt between inferior vena cava and inferior mesenteric vein.
Discussion:
Protein losing enteropathy (PLE) refers to excessive protein loss through the gastrointestinal tract. The protein loss is non-selective and includes multiple plasma proteins such as albumin, globulins, and transferrin. PLE is not a single disease but a complication of more than 60 different conditions (Levitt, 2017), including many enteric conditions (such as Crohn’s disease, coeliac disease, intestinal lymphangiectasia, Ménétrier disease, and intestinal fistula) and a variety of non-GI diseases (amyloidosis, sarcoidosis, systemic lupus erythematosus, etc.). Several mechanisms have been proposed to explain gut protein loss, such as increased lymphatic pressure (e.g. lymphangiectasia), mucosal erosion or ulceration (e.g. Crohn’s disease). PLE should be suspected in patients with hypoproteinaemia after exclusion of other causes like impaired liver function, proteinuria, malnutrition or haemodilution.
For the diagnosis of PLE, we can perform measurement of faecal alpha-1 antitrypsin clearance or radiolabelled macromolecules after intravenous injection of the tracers (e.g. I-125 albumin, Cr-51 albumin, Cu-67 ceruloplasmin). Nevertheless, it is hard to collect stool, and false-positive results may be obtained if the stool specimen is contaminated with urine.
Radionuclide scintigraphy is not only useful in establishing the diagnosis of PLE, but also for localization of the possible site of protein leakage. Several tracers have been employed to diagnose PLE. These include Tc-99m human serum albumin (Tc-99m HSA), Tc-99m dextran, In-111 transferrin; bone scan tracer Tc-99m methylene diphosphonate (MDP) has also been suggested in case reports.
HSA is a major protein component of human plasma. Tc-99m HSA can be used a variety of nuclear medicine imaging, such as imaging for protein-losing enteropathy, blood-pool imaging, sentinel lymph node mapping and lymphoscintigraphy. It is usually manufactured from a fractionation of pooled, donated human plasma. Adverse effect is seldomly reported, and possible side effects include transfusion-related infectious diseases, skin rash, tachycardia, and anaphylactic reaction.
Functional imaging utilizing Tc-99m or In-111 labelled tracers is sensitive but not specific. One systematic review of 12 studies (Khalesi et al., 2013) reported a sensitivity of 87% (81-92%) and specificity of 62% (51-72%). Use of Tc-99m labelled tracers and delayed imaging can increase the sensitivity further. False-positive results can arise from gastrointestinal bleeding, presence of free pertechnetate due to in vivo breakdown of the tracer, etc. Increased tracer activity in perfusion and blood-pool phases suggests the presence of vascular structure, and SPECT/CT in this case localises increased tracer uptake at a portosystemic shunt. Acquired portosystemic shunts are usually caused by portal hypertension in patients with liver disease.